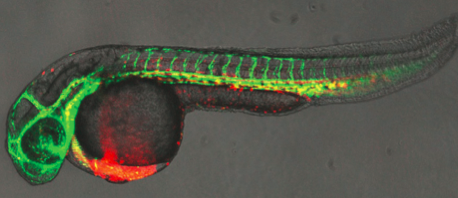
 Biologists have long wondered why the embryonic heart begins beating so early, before the tissues actually need to be infused with blood.
Biologists have long wondered why the embryonic heart begins beating so early, before the tissues actually need to be infused with blood.
Two groups of HSCI researchers from Children's Hospital Boston and Brigham and Women's Hospital —presenting multiple lines of evidence from zebrafish, mice, and mouse embryonic stem cells—provide an intriguing answer: a beating heart and blood flow are necessary for development of the blood system, which relies on mechanical stresses to cue its formation.
These studies, published in the journals Cell and Nature, together offer clues that may help in treating blood diseases such as leukemia, immune deficiency, and sickle cell anemia, and suggest new ways that scientists can make the types of blood cells a patient needs. This would help patients who require bone marrow or cord blood transplants but do not have a perfect donor match.
One team, led by Leonard Zon, MD, Chair of HSCI's Executive Committee, and Principal Faculty members Trista North, PhD, and Wolfram Goessling, MD, PhD, used zebrafish, whose transparent embryos allow direct observation of embryonic development.
Zon and colleagues discovered that compounds that modulate blood flow had a potent impact on the expression of a master regulator of blood formation, known as Runx1, which is also a known marker for the stem cells that give rise to all the cell types in the blood system.
Confirming this observation, a strain of mutant embryos that lacked a heartbeat and blood circulation exhibited severely reduced numbers of blood stem cells. Further work showed that nitric oxide, of which production is increased in the presence of blood flow, is the key biochemical regulator.
Increasing nitric oxide production restored blood stem cell production in the mutant fish embryos, while inhibiting nitric oxide production reduced the number of stem cells.
Zon and colleagues went on to demonstrate that nitric oxide production was coupled to the initiation of blood stem cell formation across vertebrate species, in mice as well as fish. "Nitric oxide appears to be a critical signal to start the process of blood stem cell production," Zon said. "This finding connects the change in blood flow with the production of new blood cells."
HSCI Executive Committee member George Daley, MD, PhD, led the second team. Intrigued by the appearance of blood progenitor cells in the wall of the developing aorta soon after the heart starts beating, they investigated the effects of mechanical stimulation on blood formation in cultured mouse embryonic stem cells.
They showed that shear stress—the frictional force of fluid flow on the surface of cells lining the embryonic aorta—increases the expression of master regulators of blood formation, including Runx1, and of genetic markers found in blood stem cells. Shear stress also increased formation of progenitor cell colonies that give rise to specific lineages of blood cells (red cells, lymphocytes, etc.). These findings demonstrate that biomechanical forces promote blood formation.
To further test these findings, Daley and colleagues studied mouse embryos with a mutation that prevented initiation of a heartbeat. These embryos had a sharp reduction in progenitor blood cell colonies, along with reduced expression of genetic markers of blood stem cells. When specific cells from the mutant embryos were exposed in vitro to shear stress, markers of blood stem cells and numbers of blood cell colonies were restored. Finally, the team showed that when nitric oxide production was inhibited, in both cell cultures and live mouse embryos, the effects of shear stress on blood progenitor colony formation were reduced.
"In learning how the heartbeat stimulates blood formation in embryos, we've taken a leap forward in understanding how to direct blood formation from embryonic stem cells in the Petri dish," Daley said.
The authors of the two papers speculate that drugs that mimic the effects of embryonic blood flow on blood precursor cells or molecules involved in nitric oxide signaling might be therapeutically beneficial for patients with blood diseases. For example, nitric oxide could be used to grow and expand blood stem cells either in the culture dish or in patients after transplantation.
