Thread-Based Diagnostics
Our laboratory explores thread as a material for fabrication or robust diagnostic devices. Thread is available in a variety of materials (e.g., Nylon, cotton, polyester, wool, polyolefin, and many others) and is inexpensive. The material can be selected to have properties appropriate to the specific application. The hydrophilicity of many kinds of thread allows movement of aqueous solutions by capillarity, without external pressure. Thread is also light, flexible, mechanically strong (both dry and wet) and comes in dimensions of smaller than 100s of microns (and thus does not require patterning to define channel sizes). Thread can be manipulated by industrialized processes (e.g., weaving and knitting) into complex structures. It thus can in principle be incorporated in a range of systems appropriate for designs of sensors that span from conventional reader-based systems to wearable sensors, microscale sensors, and for affordable sensors in applications such as food and water tests.2-4 We have developed thread-based potentiometric sensors for multiplexed analysis of electrolytes and metabolites in blood (Figure 1),4 microfluidic devices composed of pins and thread as a multiplexed electrochemical diagnostic tool (Figure 2),2 and a matrix for biomedical assays (Figure 3).3 We are developing a host of other thread-based assays to provide robust diagnostics for point of care analysis.

|
Figure 1. Functional principles and design of thread-based potentiometric electrodes. The thread-based sensors can be bundled together for multiplexed analysis of ions in the solution.4 |

|
Figure 2. Photography of a 96 well plate fabricated using embossed omniphobic RF paper and pin electrodes. The device used steel pins as reference and counter electrodes (RE and CE), and a stainless-steel pin coated with a graphite and carbon nanotube ink as working electrode (WE). |
|
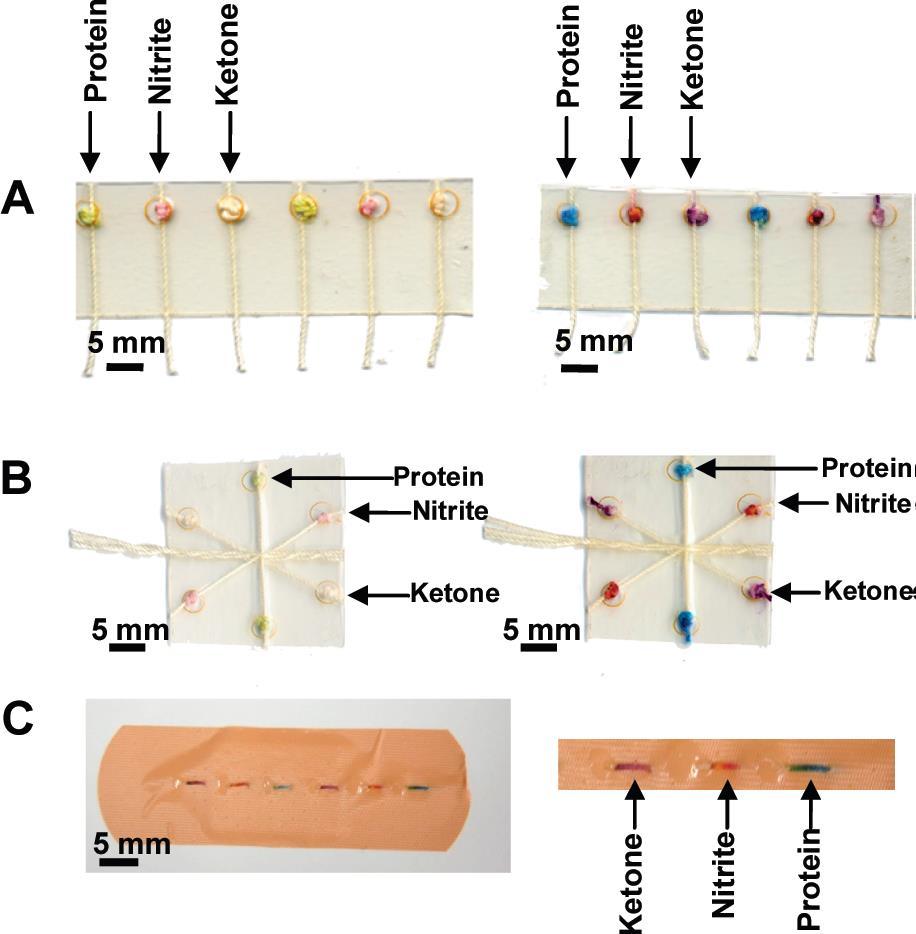
Figure 3. Colorimetric assays performed using the (A) woven array device, (B) branching device, (C) sewn array design.3 |
Paper-Based Diagnostics
Paper is one of the oldest materials humans have made. This history and widespread use of paper has led to a well-developed industry around producing paper using high-throughput printing. Paper also has the property that it absorbs and wicks liquids. By printing hydrophobic patterns on the surface of paper (by wax printing with a desktop printer or photolithography), we can define channels in paper to direct the flow of liquids.5-6 Stacking these channels and connecting them with tape allows the creation of 3D microfluidic devices (Figure 4).7 We have designed pop-up paper-based devices8 (Figure 5 and 6, add link to video showing fabrication) and electrowetting of fluidics on paper9 (Figure 7 and 8) to create complex functions from a simple material such as paper. These devices allow complex fluid handling and multiplexing. Using these processes, we have created a low-cost liver function test with a material cost of a few cents (Figure 9), and a paper-based sliding strip device for doing ELISA in resource-limited areas (Figure 10 and 11).10-15 We are developing a host of other assays to provide low-cost diagnostic information at the point of care.

|
Figure 4. Three-dimensional μPADs for running parallel assays and standards. (A) Schematic of the layers making up the device shown in B–E. (B) Photograph of the front of the dual-assay device. The sample inlets wicked the samples into the device. The dotted lines mark the edge of the device. (C) Back of the device. The reagents for colorimetric assays for glucose and proteins were prespotted in the detection zones. (D) Photograph of the device being dipped into a sample of artificial urine that contained 2-mM glucose and 40 μM BSA. The device filled with 25 μL of sample in 2 min. The glucose assay requires an additional 25 min to develop. (E) The results of the assays were displayed side-by-side for sample and control. The concentrations of glucose (Glc) and BSA in each sample of artificial urine are listed beneath the devices. (F) Top of a 4-assay device. (G) Back of the device.7 |

|
Figure 5. (a) An illustration of the fabrication process for the pop-up-EPAD (b) Schematic procedure for cutting and folding of the pop-up-EPADs. “Through cuts” require the razor to penetrate the entire thickness of the paper, while in “half cuts” the razor only scores the paper, but does not penetrate it, in order to ensure accurate folding.8 |

|
Figure 6. Pop-up paper-based electrochemical device. |

|
Figure 7. Principle of the bistable electrical valve. Schematic diagram of the operation of the valve, which is based on the principles of electrowetting on dielectric (EWOD). The gate electrode is a conductive textile covered with layers of insulator and hydrophobic coating ("electrotextile"). This electrotextile is not permeable to liquid. The application of a voltage between the liquid and the electrotextile allows liquid to flow into the paper layer underneath due to electrowetting.9 |

|
Figure 8. Design and fabrication of printed electrofluidic devices. (A) Schematic diagram of the fabrication of three-dimensional printed electrofluidic circuits. Microfluidic channels are printed using wax printing, followed by printing of electrodes (for valves and switches) and electrical wires on the paper layers. Conductive, insulated, and hydrophobic "electrotextiles" are then bonded with the paper layers using hot-lamination with polyethylene films. (B) Schematic cross-section of a fluid-to-electrical switch, and Schematic cross-section of an integrated valve, containing two microfluidic layers, and a valve layer. The valve is actuated when a voltage is applied between the liquid ("source electrode") and the electrotextile ("gate electrode") whereupon liquid can pass from the "liquid source" into the "liquid drain". (C) Circuit diagram symbols for printed microfluidic channels, printed wires/electrodes, integrated fluid-electrical switches, and valves ("electrofluidic thyristors"). |

|
| Figure 9. Schema of the paper-based AST/ALT test design and protocol. (A) Device design (lamination layer, plasma separation membrane, layers of paper). (B) Use of the device. A drop of whole blood (either a fingerstick specimen or 30 L of a specimen obtained by venipuncture) is applied to the back of the device. Red and white blood cells are filtered out by the plasma separation membrane, while plasma wicks to the five detection zones via patterned hydrophobic channels in the paper. After 15 min, the AST (top left) and ALT (top right) test zones are matched to a color guide (C) to obtain a value in U/L. Results are interpreted as being within one of three “bins” of values: <3 times (3x) ULN (defined as 40 U/L); 3–5x ULN; or >5x ULN. |

|
| Figure 10. Schematic illustration of the sliding-strip 3D µPAD. A) Top view of the (i) functional dock and (ii) sliding strip showing various parts of each component. B) Components of the sliding-strip 3D µPAD, each layer is glued using double-sided tape with holes that connect the fluidic channels. C) Operation of the sliding-strip 3D µPAD: i) while the sliding-strip is in position 1, sample is added to the inlet and washed with water, ii) the sliding-strip is moved to position 2 and water is added to the inlet to dissolve the stored detection antibodies and buffer, and to wash off excess detection antibodies, iii) the sliding-strip is moved to position 3 and water is added to the inlet to dissolve stored substrate and buffer, and to wash off excess substrate, iv) the sliding-strip is removed from the device to analyze the results visually or using a desktop scanner. D) Mechanism of operation of the sliding-strip device, the steps are analogous to those in part C.11 |

|
Figure 11. Components of a kit to be used with sliding-strip 3D µPAD in resource-limited settings: the sliding strip and the functional dock, assembled together; a 1 µL capillary to collect blood; a tube with pre-filled 1 mL of 1% w/v BSA in PBS (to be used as diluent for obtaining 1000-fold dilution of blood); a 1 mL syringe to measure out 0.1 mL of the sample; a plastic dropper to add 2 drops of water for each of the three inlets.11 |
A Universal Mobile Electrochemical Detector (uMED)
Electrochemistry is a powerful method used to quantify analytes in complex mixtures. To access the analytical power of electrochemistry at the point-of-care, a low-cost, portable electrochemical reader is necessary to read strips developed for various diagnostics. The glucose meter provides an example of an electrochemical reader designed for home use, but it is limited to only a subset of electrochemical methods (i.e., amperometry). We have created a universal Mobile Electrochemical Detector (uMED) capable of performing nearly all the functions of a benchtop potentiostat (e.g., amperometry, cyclic voltammetry, square wave voltammetry, and potentiometry) (Figure 12 and 13).16 The handheld device can be used to read commercially available electrodes as well as the paper-based electrodes we develop in the lab. In addition to providing quantitative analysis at the point-of-care, the device provides connectivity to the cloud by connecting through the headphone jack of any phone over any wireless network.

|
Figure 12. (A) An image of the uMED interfaced to a commercial glucose test-strip, and a low-end mobile phone through a standard audio cable, for transmission of data over voice. (B) A schematic of the connections and flow of data from the electrodes, through the uMED, to the remote back end. (C) A block diagram of the hardware and interconnections of the device. (D) The circuit design for the reconfigurable potentiostat. |

|
Figure 13. A demonstration of the uMED network in operation. (A and B) The local user made a blood-glucose measurement with the uMED. (C) Upon completion, the device automatically began to transmit repeated packets containing the measured value. (D) The user then connected the device to a mobile phone and placed a call to a remote Skype-in number. (E and F) The remote application i) automatically recorded the audio-based data, ii) extracted the encoded value, iii) verified that it was error-free, iv) sent an acknowledgment tone back to the uMED, and v) sent an SMS message (with relevant information) back to the local user's mobile phone. (G and H) The local user received the acknowledgment (G) and SMS (H). |
We had designed UMED to be a battery-powered, handheld device, with built-in processing capabilities, for application in resource-limited areas where access to computers or high-end electronic devices with data processing power is limited.16 The UMED interfaced with mobile phones for transmitting data to the Cloud. The interfacing was accomplished through the audio jack to ensure compatibility with all mobile devices, ranging from low-end to smartphones.16 (UWED) provides a portable potentiostat complimentary to UMED, where the experimental protocol (controlled by a smartphone or tablet) can be changed simply by the user without the need for altering the hardware or even the firmware of the device (Figure 14). The UWED also enables real-time visualization and complete transmission of the results of the measurement; this feature is helpful for troubleshooting and quality control. UWED can perform commonly used electrochemical techniques—potentiometry (POT), chronoamperometry (CA), cyclic voltammetry (CV), and square wave voltammetry (SWV) —with performance that is comparable to a commercial benchtop potentiostat.17

|
| Figure 14. Panel A and C show how the Universal Wireless Electrochemical Detector (UWED) functions. The working, reference, and counter electrodes are plugged in the UWED which is connected to a smartphone via Bluetooth Low Energy (BLE). A host program in the smartphone controls the experimental protocol, processes, visualizes, and stores the data, and transmits it to the Cloud. B shows the circuit diagram and main components of UWED. |
In some cases where analysis at the point-of-care is not possible, health workers may want to store samples and send them to a separate location for analysis. Sterile containers suitable for biological samples can be expensive. We have demonstrated that bubble wrap can provide a low-cost, sterile material for the storage and analysis of biological samples (Figure 15).18 The transparency of the bubble wrap also enables samples to be analyzed by spectroscopy without the need to transfer the sample to a different container.18

|
Figure 15. Storage and analysis of samples using bubble wrap. A test for nitrates in drinking water and for hemoglobin are carried out in chambers made from bubble wrap. |
Point-of-Care Hematology with Aqueous Multiphase Systems
Density (the mass over the volume of an object) is a property of all matter. Measuring the density of cells can provide an aggregate measure of changes taking place metabolically or morphologically. Using aqueous multiphase polymers (AMPS)—mixtures of polymers in water that spontaneously form immiscible phases—we can separate cells by density. AMPS are thermodynamically stable, can be biocompatible, and can be tuned to have specific densities in each phase.19 The interfaces between phases are molecularly sharp, and define a step in density that can collect and concentrate cells within that range of densities.
Using AMPS, we have created a simple method to enrich reticulocytes (immature red blood cells) that can be used to grow malaria species better than conventional reticulocyte purification methods (Figure 16).20 In certain cases, the ability to separate cells by density can provide a means to diagnose disease. Sickle cell disease is a genetic disease that approximately 300,000 children are born with each year. Most of these children are undiagnosed and at high risk for childhood mortality even though simple and effective interventions exist to manage the disease. The morphological changes in sickle cell disease lead to the formation of very dense erythrocytes that can be separated using AMPS. Using capillary tubes, AMPS, and portable centrifuges, we have created a low-cost, visual test for sickle cell disease that can be performed in ~15 minutes (Figure 17).21 After initial validation in the U.S., we worked with partners in Zambia to test the device in a larger population and to assess feasibility of the design in rural health centers.22

|
Figure 16. A mixture of dextran and Ficoll forms an aqueous multiphase system (AMPS) that provides a step-gradient in density. When blood layered over the system is centrifuged, cells move through the system and collect in areas based on their density. Reticulocytes are enriched at the interface of the AMPS and can be used in subsequent assays and experiments. |

|
Figure 17. A low-cost, rapid test for sickle cell disease. After centrifugation over an aqueous multiphase system, blood cells from a donor with sickle cell disease separate by density. Dense cells characteristic of sickle cell disease form a red layer at the bottom of the test. |
Another method to analyze density with minimal equipment is magnetic levitation. Using two opposing magnets, we create a magnetic field gradient. When a diamagnetic object is placed in a paramagnetic solution and within the magnetic field gradient, the paramagnetic fluid will be attracted to the highest field and will push the diamagnetic object to the point of lowest field in the middle of the two magnets. This force is opposed by the buoyant force which drives the object to the top or bottom of the solution. The balance of the magnetic and buoyant forces results in a stable levitation height within the paramagnetic fluid that is a function of density.23
Using antigens immobilized onto the surface of polymeric beads, we have created a density-based ELISA. When the beads are exposed to a sample, antibodies specifically bind to the antigens. Anti-human antibodies conjugated with gold particles then bind to the captured antibody. We then chemically deposit silver or gold onto the beads. The deposition of metal leads to a change in density of the bead that indicates the amount of antigen that was captured.24 Using parallel vials, we can perform a multiplexed density-based ELISA (Figure 18).

|
| Figure 18. Multiplexed immunoassay for syphilis and Hepatitis C (HepC). When beads with a specific antigen immobilized on their surface are exposed to a sample with the corresponding antibody conjugated to gold, the bead increases density through the deposition of gold. Positive and negative controls are provided by beads coated with BSA and human IgG, respectively. At the beginning of the assay, all beads are at the top of the device (A). After exposure to serum containing various antibodies, a normal sample (B) causes only the positive control bead to fall. A sample positive for HepC causes the corresponding bead to fall (C), and a sample positive for both HepC and syphilis, causes both test beads to fall (D). |
