Mucosal Immunology
Mucosal surfaces constitute the inner lining of the body and cover sites that include the digestive, respiratory, and the urogenital tract. While some functions, such as nutrient absorption in the digestive tract, are unique of certain mucosal sites, all mucosal surfaces have in common a constant exposure to foreign pathogens. Thus, it is expected that immune responses are well developed in the mucosae. To assure immune surveillance, immune cells must acquire the expression of a set of membrane proteins (integrins and chemokine receptors) that allows them to home to mucosal sites. One of the research interests of the lab involves the characterization of processes, such as mucosal imprinting of lymphocytes, that define homing to mucosal sites. One of our goals is to understand which factors influence mucosal imprinting of immune cells, and how these factors can be used for vaccine formulations against mucosal pathogens. To develop these studies, we use mucosal pathogens such as rotavirus, Listeria, Chlamydia, influenza virus, etc., as infection models. Mucosal surfaces constitute the inner lining of the body and cover sites that include the digestive, respiratory, and the urogenital tract. While some functions, such as nutrient absorption in the digestive tract, are unique of certain mucosal sites, all mucosal surfaces have in common a constant exposure to foreign pathogens. Thus, it is expected that immune responses are well developed in the mucosae. To assure immune surveillance, immune cells must acquire the expression of a set of membrane proteins (integrins and chemokine receptors) that allows them to home to mucosal sites. One of the research interests of the lab involves the characterization of processes, such as mucosal imprinting of lymphocytes, that define homing to mucosal sites. One of our goals is to understand which factors influence mucosal imprinting of immune cells, and how these factors can be used for vaccine formulations against mucosal pathogens. To develop these studies, we use mucosal pathogens such as rotavirus, Listeria, Chlamydia, influenza virus, etc., as infection models.
NK Cell Memory
Natural killer (NK) cells were discovered over 40 years ago as a population of lymphocytes that could lyse sheep red blood cells and tumor cells “naturally” (i.e.without requiring prior activation). Traditionally, NK cells have been classified as innate immune cells that recognize their targets through a variety of germqline-encoded receptors. However, our laboratory discovered that a subset of NK cells has the capacity to mediate long-lived, antigen-specific adaptive immunity (Paust and von Andrian, 2011). We are currently employing a wide variety of investigative lines, including genetic, bioinformatic, cellular, and biochemical approaches to identify the molecular mechanism(s) underlying NK cell-mediated adaptive immunity.
Ready to Recruit: Leukocyte Trafficking
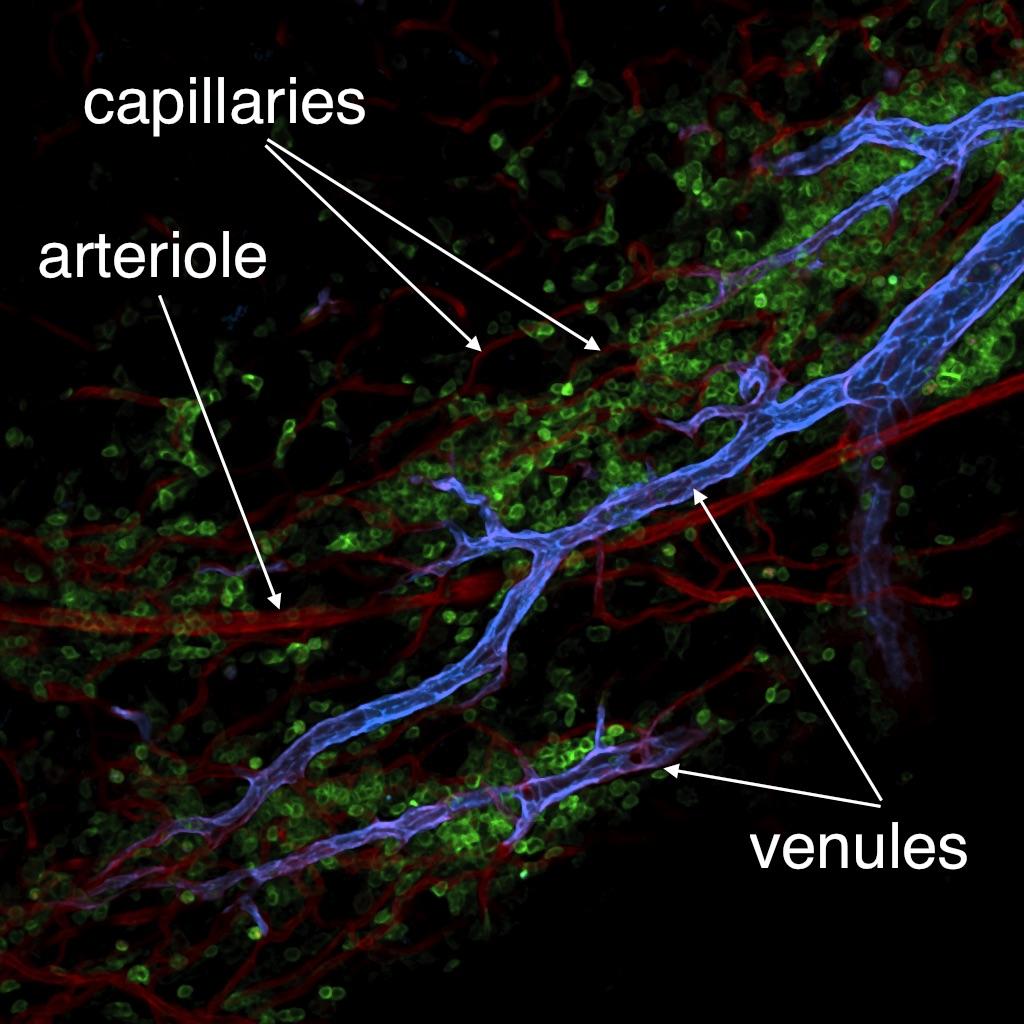
We have a long-standing interest in elucidating the mechanisms and consequences of immune cell migration from blood into tissues. A better understanding of this process holds great promise for the discovery of new therapeutic modalities to treat a wide range of pathologies from inflammatory diseases to cancer. A well-established fact is that leukocyte interactions with microvessels are restricted to postcapillary and collecting venules, whereas capillaries and arterioles usually do not support significant leukocyte adhesion. The differentiation program(s) that enable(s) leukocyte recruitment by venular endothelial cells, but prohibit(s) recruitment by non-venular capillary and arteriolar endothelium, are unknown. To address this issue, we have recently shown that a newly developed monoclonal antibody against DARC (ACKR1) selectively recognizes venules in normal murine tissues. Using this mAb, we have shown that primary venules and non-venules from select organs can be purified and compared at the transcriptome and proteome level.
Viral Immunology
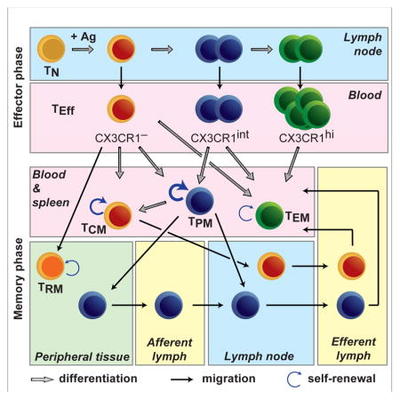 Viral acute infections induce pathogen-specific T cell activation and differentiation into heterogeneous effectors that give rise to different memory subsets. We are interested in untangling the cell-fate decisions and lineage relationships that underlie the effector-to-memory transition as well as the maintenance of memory subsets. In this regard, we have identified the chemokine receptor CX3CR1 as a key marker to distinguish three distinct CD8+ T effector and memory subsets (negative, intermediate and high) that differ in phenotypic characteristics, trafficking properties and specialized functions. This finding has allowed us to reformulate the long-held paradigm of central (CX3CR1neg) vs. effector (CX3CR1high) memory cells by including now the peripheral memory (CX3CR1int) cells that are chiefly responsible for the global surveillance of non-lymphoid tissues (Gerlach et al., Immunity 2016).
Viral acute infections induce pathogen-specific T cell activation and differentiation into heterogeneous effectors that give rise to different memory subsets. We are interested in untangling the cell-fate decisions and lineage relationships that underlie the effector-to-memory transition as well as the maintenance of memory subsets. In this regard, we have identified the chemokine receptor CX3CR1 as a key marker to distinguish three distinct CD8+ T effector and memory subsets (negative, intermediate and high) that differ in phenotypic characteristics, trafficking properties and specialized functions. This finding has allowed us to reformulate the long-held paradigm of central (CX3CR1neg) vs. effector (CX3CR1high) memory cells by including now the peripheral memory (CX3CR1int) cells that are chiefly responsible for the global surveillance of non-lymphoid tissues (Gerlach et al., Immunity 2016).
Neuroimmunology
The immune system has evolved to protect the host from infectious agents, parasites and tumor growth, and to assure the maintenance of homeostasis. Similarly, the primary function of the somatosensory branch of the peripheral nervous system is to provide sensory information about the environment, allowing the organism to react to and avoid situations, which could otherwise have deleterious effects. Consequently, it follows that the two systems should cooperate and, indeed, multiple recent studies have revealed an exciting, context-specific interaction between the immune system and the peripheral nervous system in multiple tissues.
To gain detailed insights into the underlying molecular and cellular mechanisms involved in this neuroimmune crosstalk, we are using a wide variety of approaches ranging from biochemistry and in vitro culture systems to state of the art imaging and single-cell RNA sequencing techniques. Our ultimate goal is to broaden our knowledge of the neuroimmune interactions and to open new, previously unappreciated avenues of research.
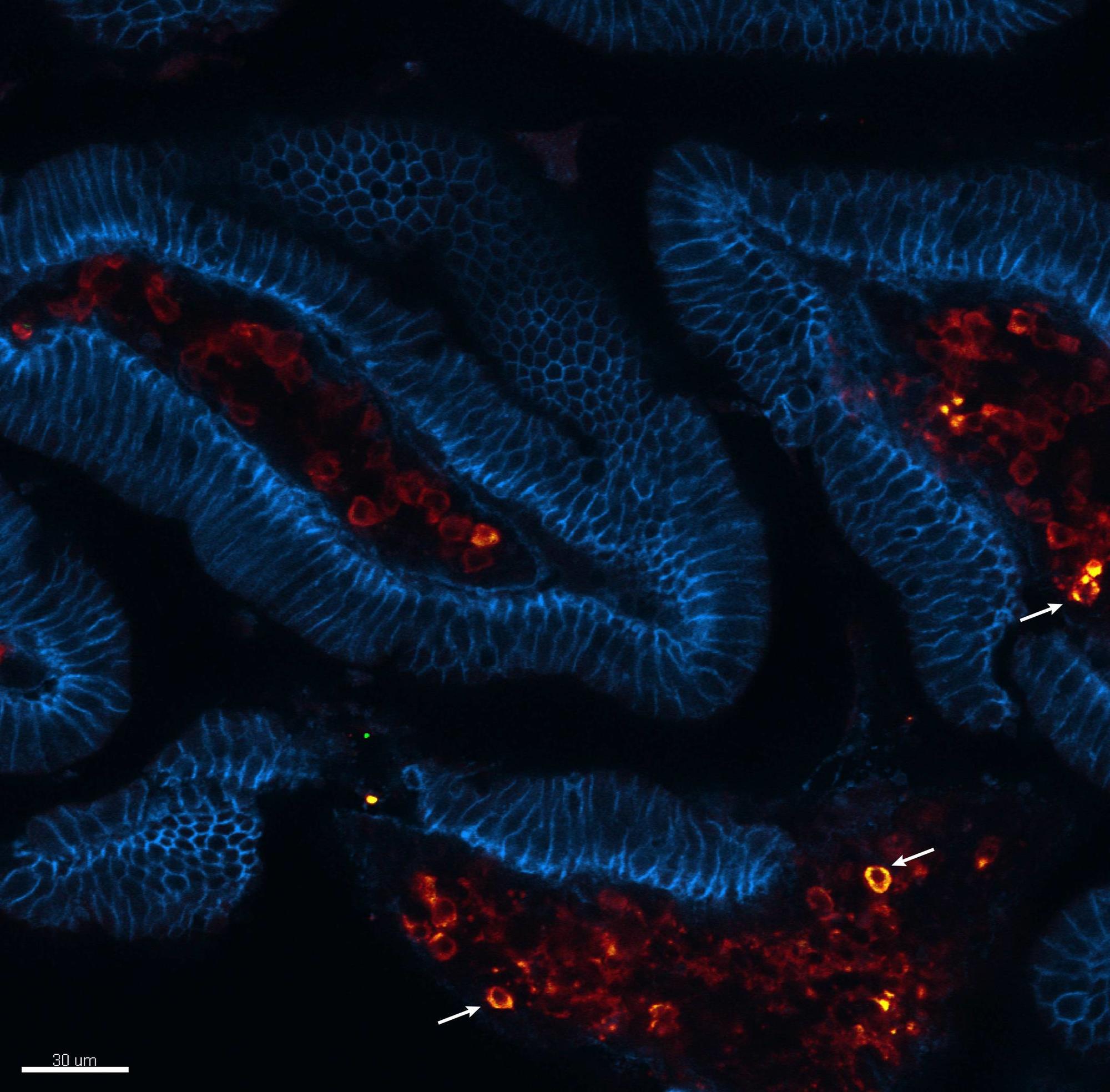
 Viral acute infections induce pathogen-specific T cell activation and differentiation into heterogeneous effectors that give rise to different memory subsets. We are interested in untangling the cell-fate decisions and lineage relationships that underlie the effector-to-memory transition as well as the maintenance of memory subsets. In this regard, we have identified the chemokine receptor CX3CR1 as a key marker to distinguish three distinct CD8+ T effector and memory subsets (negative, intermediate and high) that differ in phenotypic characteristics, trafficking properties and specialized functions. This finding has allowed us to reformulate the long-held paradigm of central (CX3CR1neg) vs. effector (CX3CR1high) memory cells by including now the peripheral memory (CX3CR1int) cells that are chiefly responsible for the global surveillance of non-lymphoid tissues (Gerlach et al., Immunity 2016).
Viral acute infections induce pathogen-specific T cell activation and differentiation into heterogeneous effectors that give rise to different memory subsets. We are interested in untangling the cell-fate decisions and lineage relationships that underlie the effector-to-memory transition as well as the maintenance of memory subsets. In this regard, we have identified the chemokine receptor CX3CR1 as a key marker to distinguish three distinct CD8+ T effector and memory subsets (negative, intermediate and high) that differ in phenotypic characteristics, trafficking properties and specialized functions. This finding has allowed us to reformulate the long-held paradigm of central (CX3CR1neg) vs. effector (CX3CR1high) memory cells by including now the peripheral memory (CX3CR1int) cells that are chiefly responsible for the global surveillance of non-lymphoid tissues (Gerlach et al., Immunity 2016).

